patients would choose to continue receiving HyQvia*

to choice of
where your
patients get their
treatment
HyQvia may be reimbursed across multiple sites of care1*
Give your patients the option of infusing in-center/in-office, or at home (HCP-supported or self-infused after appropriate training).
In-center/in-office
HCP-supported administration may be more desirable for patients who:
- Are not comfortable or unable to self-administer2
- Have venous access issues or may require port and could benefit from subcutaneous option2,3
- Prefer infusions to be handled by a HCP2
- Lack the organizational skill, dexterity or drive to reliably self-administer2,3
- Prefer administration where they already receive care
At home
Self-infusion or HCP/caregiver-supported administration may be preferable for patients who:
- Have logistical issues attending or live far away from an infusion center or a clinic3
- Need to fit infusions around work/home life4
- Want more independence and flexibility3,4
- Have venous access issues or may require a port and could benefit from a subcutaneous option2,3
- Would benefit from HyHub™ and HyHub™ Duo for HyQvia—infusion trays designed to help simplify the infusion preparation process for your patients 17 and older, as prescribed, without the use of transfer needles by reducing the number of steps required to prepare Hy and IG5-7†
Select Important Information for Healthcare Providers:
Indications for Use: HyHub/HyHub Duo are indicated for patients 17 years of age and older to allow HyQvia [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase] to be transferred from vials without using a needle, as prescribed, in a home environment or clinical setting.
Contraindications:
- Do not use HyHub/HyHub Duo with a pooling bag.
- Do not connect HyHub/HyHub Duo to a syringe driver infusion pump.
Click here for additional Important Information for HCPs.
Patients should not use HyHub/HyHub Duo at home until receiving instructions and training from a healthcare provider. For safe and proper use of HyHub/HyHub Duo, please refer to the complete Instructions for Use included with the devices.
HCP=healthcare professional.
*Patients can receive HyQvia treatment at an infusion center, in hospital, or at home. It can be given by an HCP, self-administered after appropriate training, or given by a trained caregiver. A choice of home administration must be a joint decision between HCP and patient; patients cannot make this decision themselves.1
†Compared to preparing with a pooling bag using 2-4 dual-vial units (DVUs).
Patient choice
Patients’ choice of administration methods8
In a self-administered questionnaire using a non-validated scale during ADVANCE-1, patients were asked “Now that you’ve experienced different methods to receive your immunoglobulin therapy, would you choose to continue receiving your treatment using the study drug?” The response was the following:
~9/10
*In ADVANCE-1, 86.7% (85/98) of patients chose to continue receiving their treatment using the study drug. In ADVANCE-1, questionnaire was administered at both baseline and the final visit. Number of subjects within both treatment groups in the ADVANCE-1 MITT Analysis Set who had available data during treatment.
Administration experience
Over the course of ADVANCE-1 and ADVANCE-3,* administration remained approximately the same.9,10
Most with
Every 4 weeks
administrative frequency
~ 2 hours infusion time
Most with
2 infusion sites
| Average Dose | Administrative Frequency every 2, 3, or 4 weeks | Infusion Time | Infusion Sites 1, 2, or 3 infusion sites | |
|---|---|---|---|---|
| ADVANCE-19 | 85 g† (1.1 g/kg) | 88% dosing interval every 4 weeks 11% every 3 weeks 1% every 2 weeks | ~ 2 hours Median (range): 116.5 min (65, 259) | 86% administration with 2 infusion sites (n=114/132) 10% with 1 site (n=13/132) 4% with 3 sites (n=5/132) |
| ADVANCE-3 FINAL10 | 64 g‡ (0.9 g/kg) | 88.2% dosing interval§ every 4 weeks 11% every 3 weeks data not available for every 2 weeks | ~ 2.25 hours Median (range): 135.5 min (65, 8) | 92.3% administration with 2 infusion sites (n=78/85) 2.7% with 1 site (n=2/85) 5% with 3 sites (n=4/85) |
| Average Dose | Administrative Frequency every 2, 3, or 4 weeks | Infusion Time | Infusion Sites 1, 2, or 3 infusion sites | |
|---|---|---|---|---|
| ADVANCE-19 | 85 g† (1.1 g/kg) | 88% dosing interval every 4 weeks 11% every 3 weeks 1% every 2 weeks | ~ 2 hours Median (range): 116.5 min (65, 259) | 86% administration with 2 infusion sites (n=114/132) 10% with 1 site (n=13/132) 4% with 3 sites (n=5/132) |
| ADVANCE-3 FINAL10 | 64 g‡ (0.9 g/kg) | 88.2% dosing interval§ every 4 weeks 11% every 3 weeks data not available for every 2 weeks | ~ 2.25 hours Median (range): 135.5 min (65, 8) | 92.3% administration with 2 infusion sites (n=78/85) 2.7% with 1 site (n=2/85) 5% with 3 sites (n=4/85) |
fSCIG=facilitated subcutaneous immunoglobulin.
*A single-arm, open-label extension safety study.1
†The mean monthly dose equivalent (averaged per patient at 4.35 weeks/month) for patients receiving fSCIG 10% or placebo was 85.4 g or 84.8 g, respectively, equal to 1.1 g/kg for the fSCIG 10% group or 1.0 g/kg for the placebo group. For patients receiving fSCIG 10% or placebo, the median (range) monthly dose equivalent was 82.6 g (27–217 g) or 69.6 g (27–217 g), respectively.9
‡Patients who received fSCIG 10% in ADVANCE-CIDP 1 continued to receive this therapy at the same dose (mean monthly dose equivalent of 1.1 g/kg, with maximum 4-weekly administration) until relapse or study end. The median 4-weekly dose equivalent (averaged per patient) was 64.0 g (28.0-200.0).10
§Patients could adjust their interval after 12 weeks while maintaining the same monthly dose.10
Calculating the right dose and schedule of maintenance therapy for your adult patients with CIDP
HyQvia gives you and your patients administration schedule and dosing options1*
Dosing and ramp-up calculator
With just three data inputs, you can calculate your patient's dosage and various frequency options for HyQvia administration. Enter your patient’s current IVIG dose, their current IVIG frequency, and their planned HyQvia frequency, and the tool will take care of the rest.
Use this tool to calculate your patient’s dosage and various frequency options for HyQvia administration.
For your patient who is:
Est. time to infuse:
US-HYQ-1483v1.0 03/25
This tool is intended to estimate the full infusion time after the first 2 initial doses and includes a 10-minute wait between hyaluronidase and immune globulin infusion. The calculation for hyaluronidase used the push method of 2 mL/minute. Please refer to CIDP infusion rate in section 2.2 of USPI for full details as well as details for longer infusion time in the first 2 doses. If the pump method is used to infuse hyaluronidase, please ensure that the maximum rate is ≤300 mL/hr when using 3 sites.
Please ensure to adjust the infusion rate based on patient tolerability. For the IG component of HyQvia, the duration of infusion is based on the fastest infusion for each step, including the initial infusions at 5-minute intervals (can be increased up to 15-minute intervals). All dosing options can be found in section 2 of the USPI.
See Full Prescribing Information for additional dosage recommendations.
When administering HyQvia, there is a “ramp-up” period. A “ramp-up” period can take 4-9 weeks, depending on the dosing interval and tolerability.1 Use the calculator below to see how HyQvia ramps up for your patient.
Week 1
no infusion
Week 2
22.5 g
(25%)
Week 3
22.5 g
(25%)
Week 4
45 g
(50%)
Week 5
Week 6
67.5 g
(75%)
Week 7
Week 8
Week 9
90 g
(100%)
Week 10
Week 11
Week 12
6 months
This tool is intended to estimate the ramp-up period for the full infusion volume of HyQvia. Please refer to CIDP infusion rate in section 2.2 of USPI for full details on infusing over several days in case the total dose is >1200 mL (120 g) for subjects weighing ≥40 kg or 600 mL (60 g) for subjects weighing 40 kg.
See Full Prescribing Information for additional dosage recommendations.
This dosing and ramp-up calculator is meant to be used as a tool to help you make informed decisions about dosing. Takeda is not making dosing recommendations.
Use the estimated dose and frequency in our ramp-up calculator to see how HyQvia ramps up to the full dose.
- Dose and dosing frequency can be adjusted based on the individual patient’s clinical response1
- The typical dosing interval for HyQvia in the clinical trial was 4 weeks. For patients with IVIG dosing >4 weeks, the dosing schedule can be converted to 3 or 4 weeks while maintaining the same monthly equivalent IgG dose1†
CIDP=chronic inflammatory demyelinating polyneuropathy; IVIG=intravenous immune globulin.
*Patients can receive HyQvia treatment at an infusion center, in-office or at home. It can be given by an HCP, self-administered after appropriate training or given by a trained caregiver. A choice of home administration must be a joint decision between HCP and patient; patients cannot make this decision themselves.1
†Variations in the dosing interval of up to ±7 days or monthly equivalent dose amount of up to ±20% between the patient’s IgG infusions are considered a stable dose.1
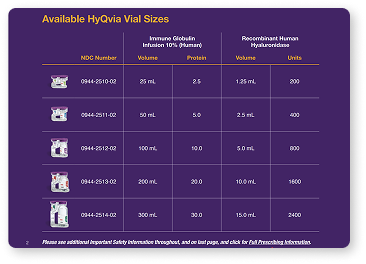
HyQvia is available in several vial sizes to accommodate your patient's needs
Recommended HyQvia infusion dose ramp-up schedule when transitioning from stable doses of IVIG1*
Depending on the treating physician’s discretion, in patients who tolerate the first two infusions well, subsequent infusions may be administered by gradually increasing doses and decreasing dose intervals, considering the volume and total infusion time.1
Transitioning from IVIG: Ramp-up period up to 4-9 weeks depending on dosing interval and tolerability1
| HyQvia Dosing Schedule | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | 6 months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q4 weeks1 | X | 25% | 25% | 50% | X | 75% | X | X | 100% | Full dose every 4 weeks | |||
| Q3 weeks3 | X | 33% | 33% | 67% | X | 100% | Full dose every 3 weeks | ||||||
| Q2 weeks3 | X | 50% | 50% | 100% | Full dose every 2 weeks |
Administer the 1st infusion two weeks after the last IVIG infusion1
*Clock starts one week after the last IVIG dose is administered. Week 1 is the week that starts one week after the last IVIG dose.1
Review safety information
Safety Information, including contraindications and other specific warnings and precautions to consider when prescribing and monitoring patients treated with HyQvia.
Varied product administration characteristics
HyQvia enables SC administration of a full dose up to once monthly,* with an average infusion time of approximately 2 hours.1,11
IV=intravenous; SC=subcutaneous.
*Every 2, 3, or 4 weeks.1
‡Patients can receive HyQvia treatment at an infusion center, in hospital, or at home. It can be given by an HCP, self-administered after appropriate training, or given by a trained caregiver. A choice of home administration must be a joint decision between HCP and patient; patients cannot make this decision themselves.1
Based on information from product labeling. No head-to-head studies have been conducted.
No representation of comparative safety, efficacy, or FDA-approved indications should be made from these data.
†See GAMMAGARD LIQUID Important Safety Information including Boxed Warnings regarding Thrombosis, Renal Dysfunction, and Acute Renal Failure, and full prescribing information.
Methods to prepare HyQvia
When selecting and preparing a pump for administering HyQvia, the following criteria should be taken into consideration:
- The IG component of HyQvia must be administered using an infusion pump capable of infusing a patient’s dose up to every 3-4 weeks and at an infusion rate of up to 300 mL/h/site1
- The selected pump should be indicated for subcutaneous (SC) use1
- While part of the fully assembled administration system, the pump must have the ability to titrate the flow rate up or down, as required to improve tolerability1
- The pump’s maximum occlusion alarm setting should be at least 11.6 psi19
- HyHub and HyHub Duo require administrative sets equipped with a luer lock, slide clamp, and needleless Y-site20,21
Find out more here.
Learn about infusion
with a peristaltic pump
Watch our step-by-step infusion videos below for healthcare professionals. You can also download our Dosing & Administration Guide on how to infuse HyQvia with a peristaltic pump.
Watch our nurse infusion video for HyQvia
- 00:00 Say Hy to Infusing with a Peristaltic Pump
- 05:05 Hy1: Get ready
- 06:32 Hy2: Prepare the Hy
- 09:56 Hy3: Prepare the IG
- 13:15 Hy4: Infuse
- 18:38 Hy5: Finish up
Hy=hyaluronidase; IG=immune globulin.
- 0:00 Nurse training video for HyHub
- 5:55 hy1: Gather & prepare for infusion
- 15:02 hy2: Prepare the infusion site
- 16:05 hy3: Infuse hyaluronidase, or Hy
- 17:48 hy4: Infuse immune globulin, or IG
- 20:01 hy5: Complete & log infusion
Nurse: This training video shows nurses how to teach patients or their caregivers how to infuse HyQvia using HyHub or HyHub Duo, two convenient infusion trays for patients 17 years of age or older with either Primary Immunodeficiency (PI) or Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). Both patients and caregivers will follow the same steps in the exact same way.
Nurse: HyHub and HyHub Duo important information for health care providers. Intended use: HyHub and HyHub Duo or stand-alone, single-use disposable vial access devices. Indications for use: HyHub and HyHub Duo are indicated for patients 17 years of age and older to allow HyQvia [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase] to be transferred from vials without using a needle as prescribed in a home environment or clinical setting.
Nurse: Contraindications. Do not use HyHub or HyHub Duo with a pooling bag. Do not connect HyHub or HyHub Duo to a syringe driver infusion pump. Selected information for patients. HyHub and HyHub Duo are for single use only. Even if all docks are not used during a single infusion, re-use will increase risk of infection.
Nurse: Patients should always use a new HyHub and HyHub Duo for each infusion. Only use HyHub and HyHub Duo when patients are ready to administer HyQvia. Patients should not use HyHub and HyHub Duo at home until receiving instructions and training from a health care provider. HyQvia is the only medication that may be used with HyHub and HyHub Duo.
Nurse: Patients should not exceed the maximum infusion volume per infusion site or infusion rate, as indicated in the HyQvia prescribing information. For safe and proper use of HyHub and HyHub Duo, please refer to the complete instructions for use included with the devices. For information about HyQvia, including warnings for thrombosis. Please see Prescribing Information for HyQvia.
Nurse: Indications. HyQvia is indicated for the treatment of primary immunodeficiency (PI) in adults and pediatric patients two years of age and older, and for chronic inflammatory demyelinating polyneuropathy (CIDP) as maintenance therapy to prevent relapse of neuromuscular disability and impairment in adults. HyQvia is for subcutaneous use only. Important safety information. Warning thrombosis. Thrombosis may occur with immune globulin (IG) products, including HyQvia. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity, and cardiovascular risk factors.
Nurse: Thrombosis may occur in the absence of known risk factors for patients at risk of thrombosis, administer HyQvia at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
Nurse: Please see additional important safety information throughout this video. Accompanying full prescribing Information may be found on HyQvia HCP.com. Please discuss any questions or challenges with your patient's health care provider (HCP).
Nurse: It's important to note that the instructions in this video are the same for both HyHub, which holds up to four dual vial units, or a maximum of 120g of IG, and HyHub Duo, which holds up to two dual vial units or a maximum of 60g of IG. We'll be using the four dock tray for this training.
Nurse: Please be aware that your patient could receive either HyHub or HyHub Duo, depending on their dose and what their specialty pharmacy has in stock. Hi, I'm Christina and I'm a nurse. Today I'm going to show you how to teach patients to self-administer after appropriate training, HyQvia, using the HyHub infusion tray designed to help simplify the infusion process without the use of transfer needles.
Nurse: You can explain to patients that because this tray replaces the pooling bag, they can prepare for their HyQvia infusion without the use of transfer needles. Please note that most patients will have received their HyHub tray and infusion supplies in the mail from their specialty pharmacy before you visit them in their home. It's normal for your patients to be overwhelmed or nervous at the beginning of infusion training.
Nurse: Reassure them that you will be giving them detailed step by step instructions, and will explain what everything in their box of supplies is for. Let's begin. The HyQvia infusion with HyHub is broken down into five steps. We call them hy one, hy two all the way to hy five. This entire process is known as hy five.
Nurse: Now we're at hy one. Checking the HyHub tray and vials, gathering all the supplies, and preparing for infusion. Make sure to start with a clean work surface and begin by reviewing the supplies. First, take out the HyHub tray, which should be packed right on top. A printed instructions for use (IFU) document will be included in the tray packaging.
Nurse: Here are all the supplies your patient will receive from their specialty pharmacy. Start by pointing out Hyaluronidase or Hy. Then show them the IG vials. The size and number of the vials may vary depending on the prescribed dose. Then show your patient the pump and the pump tubing. Please note that pump tubing is for single use with the peristaltic pump and HyHub infusion tray.
Nurse: Then continue to point out the syringe, the subcutaneous needle set, and finally the sharps container. Explain that this is where they will dispose of the needle set and syringe when they are done. The rest of the box contains gloves, a prefilled saline syringe or dextrose 5%, and water. There's tape, a bandage, gauze and alcohol swabs to clean the tops of the vials and their body where they infuse.
Nurse: And last but not least, your PI patients will get a wellness journal. This is where they keep track of all of their treatments, any reactions after each infusion and write down questions for their health care provider. If they did not receive a wellness journal in their shipment and would like one, PI patients can request one through Takeda patient support.
Nurse: CIDP patients can order one through HyQvia.com. Your patients might feel like it's a lot at first, but remind them that the process will become more familiar as they learn what everything is. An infusion mat where your patients can lay out their supplies is also available in the patient starter kit. The mat has a QR code that leads to an infusion video if they are in need of reminders later.
Nurse: It's also good to remind them that the step by step guide, which comes with the supplies, is a great go to resource, as is their specialty pharmacy if they have any questions. Okay, let's get started. Whenever you teach a patient how to self-administer an infusion, it's important to remember that the hy comes before the immune globulin infusion. To help patients remember, we teach them to say hy before IG.
Nurse: It's just like starting a conversation. You always start with hi. Make sure your patients know to allow vials to reach room temperature. This may take up to 60 minutes or longer. It's important to tell your patients never to apply heat or put their medication in the microwave. The vials should come to room temperature naturally. Once that's established, have your patient check the expiration date.
Nurse: Advise them not to use vials if the expiration date has passed, or if the purple protective cap is missing or broken. Next, tell them to always make sure they have been given the correct dose prescribed by their HCP. Point out the two vial labels. One is recombinant human hyaluronidase. We call that hy for short. This other one is immunoglobulin 10% or IG for short.
Nurse: Tell your patients not to shake the vials. Have them look at the color. The hy should be clear and colorless, and the IG should be clear and colorless or pale yellow. Neither should be cloudy nor have any floating particles. Remind your patients to wash their hands thoroughly with soap and water and let them dry before starting the infusion process.
Nurse: Wearing gloves is optional, but they should wear them if that is what has been recommended by their HCP. From here, look at HyHub and its packaging with your patient. Have them check the expiration date which is located right on the packaging. Never use an expired HyHub or other expired infusion materials. Have your patient open and inspect the supply packaging.
Nurse: They should not use HyHub or its components if they appear to be broken, missing or expired. This may cause infection, leaks from the product or other hazardous conditions that could result in serious injury. If they observe any of that, tell them to contact their HCP or specialty pharmacy as soon as possible. The HyHub infusion tray is numbered and color coded to help with following the instructions.
Nurse: The tubing is built inside and these are the hy and IG connectors, which will be used to transfer the medication from the vials. This is where the vials dock in. Your patient will remove the purple caps from the dual vial units. Usually the blue vial caps underneath come off with the purple caps, but if they do not remove them manually. Instruct your patients to peel off the labels from the IG vials and place them in their infusion log.
Nurse: Have your patient clean the tops of all the vials using a separate alcohol swab for each, and give the vials 30 seconds to dry. Have them start with the hy vials first and then do the IG vials. Again, hy before IG. Now that they're clean, have your patient dock the vials into the HyHub infusion tray. The numbers on the covers show the order for adding the vials.
Nurse: Up to four dual vial units can be added, but as you can see, this patient only needs to use two. Keep in mind, HyHub Duo has two docks and can hold up to two DVU’s. The following steps will be the same for each set of vials, whether they are using one, two, three, or four. Your patient will remove the HyHub cover, being careful not to touch or remove anything under it.
Nurse: Touching anything under the cover will increase the risk of contamination and interfere with the normal function of the HyHub infusion tray. Starting with dock one, your patient will insert each dual vial unit into a numbered dock with the stoppers facing down. It is important to insert the DVU’s in the order of the dock numbers.Push down, so that the dual vial unit locks in place.
Nurse: You may hear a click, which is an indicator that the dual vial unit is locked in place. If your patient is using more than one dual vial unit, have them repeat dual vial units and insert them in order into docks two, three, and four as needed. Depending on the infusion dose and number of dual vial units, they may not use all docks.
Nurse: If your patient uses more than four dual vial units, instruct them to contact their healthcare professional for instructions. Once the vials have been locked into place, they should not be removed from the dock. The vials shouldn't be rotated after docking either. This may cause leaks or damage to the spikes. Next, your patient will push in and twist the syringe to the purple hy connector.
Nurse: Have them remove the hy connector from HyHub by pulling it gently away from the infusion tray. That will reveal the tubing. Then remove the syringe from the packaging. The syringe size may vary depending on dose. To avoid accidental contamination, it should never touch the tip of the hy connector. Before connecting the syringe to the hy connector, tell your patient to make sure to remove all the air from the syringe by pushing down on the syringe plunger.
Nurse: Then instruct your patient to attach the syringe to the end of the purple hy connector by pushing in and twisting the syringe onto the hy connector. Next, they should pull back on the syringe’s plunger to draw all of the hy up into the syringe without pushing any air into the hy tubing. Then they should remove the syringe from the hy connector with a twist to the left.
Nurse: More than one syringe may be needed to administer the complete dose, depending on the total volume to be administered. Okay, all the necessary hy is in the syringe. It's time to attach it to the needle set. But first have them point the syringe tip up and push the syringe’s plunger up to remove air. Your patient should remove the cap from the end of the needle set tubing. Then twist the syringe filled with hy until the tubing connected to the end of the needle set.
Nurse: They will need to fill the needle set tubing with hy until it reaches the needle wings. Instruct them not to push the hy beyond the needle wings. Have your patient set aside the syringe and needle set for their infusion.
Nurse: Next, to prepare the pump and pump tubing to infuse IG, your patient will need to attach the pump tubing to the orange IG connector on the HyHub infusion tray. They'll twist the pump tubing onto the IG connector to lock it in place. Remind them not to touch the tip of the IG connector. Have your patient insert the pump tubing into the pump itself.
Nurse: The top flips open and the tubing fits inside. The tubing should be filled according to the pump manufacturer's instructions. Now that you've helped your patient prepare the HyHub infusion tray, it's time for hy two preparing the infusion site. Ask your patient where they would like their infusion site to be, either in the middle to upper abdomen or the thighs.
Nurse: Bony areas like the ribs, visible blood vessels, scars, and any areas of inflammation or infection should be avoided. Explain that it's important to rotate sites by choosing opposite sides of the body for each new infusion. If their HCP tells them to infuse in two sites, they should select sites on opposite sides of the body. If using three sites, the site should be ten centimeters apart.
Nurse: For patients who have a wellness journal, instruct them to use it to write down their infusion sites. Show your patient how to use a new alcohol swab to clean the abdomen and allow it to dry, at least 30 seconds. If they are using more than one infusion site. They will need to follow these steps again. Okay, we're at hy three, infuse hy.
Nurse: Now that they've prepared the infusion site, have your patient remove the needle cover and firmly grasp and pinch at least one inch of skin between two fingers. Have them hold the needle set so that the needle is pointed at a 90 degree angle, straight at where they want it to go in. The needle should be inserted with a rapid motion straight into the skin.
Nurse: Instruct your patient to use some sterile tape to tape down the needle wings and secure the needle in place. Remember, they'll want to repeat these steps if they're using more than one infusion site. Now let's do a needle check with a syringe filled with hy to make sure the placement is good by pulling back on the syringe’s plunger to check for blood return and the tubing of the needle set.
Nurse: If your patient sees blood in the tubing, instruct them to remove and discard the subcutaneous needle and repeat hy two and hy three with a new subcutaneous needle and infusion site. After the needle check secure the needle set by applying a sterile, clear dressing that should help to hold it in place if they need to move around. We want to infuse the hy manually for the prescribing information at an initial rate per infusion site of approximately 1 to 2ml per minute, and increase as tolerated.
Nurse: If your patient is using more than one infusion site, have them divide the hy equally between sites. After infusing all the contents in the syringe. Your patient can remove the empty hy syringe from the needle set. Remove the sterile cap on the pump tubing and twist the pump tubing filled with IG to the right onto the same needle set.
Nurse: Your patient is ready to infuse IG. This is hy four. Here's where the peristaltic pump comes into play. Make sure the pump tubing is properly secured to the needle set. Start the IG infusion within ten minutes of completing the infusion of hy. Verify that the infusion pump is programed according to the prescribed infusion rates and manufacturer's instructions.
Nurse: If using more than one infusion site, do not exceed pump infusion rate of 600ml per hour. For example, 300ml per hour per site for two sites or 200ml per hour per site for three sites. Start the pump to infuse the IG from all docked IG vials. The pump will make a little humming sound. That's good. It means it's working.
Nurse: Never rotate the vials during the infusion. This may cause leaking or damage to the spikes. When nearing the end of the infusion, it's good to check in with your patient and remind them to keep the infusion tray flat to prevent air from entering the system. It is also a good time to explain that a temporary raised area around the infusion site is likely.
Nurse: Here's what I usually say. You may have a soft, wide, swollen area around your infusion site due to the amount of fluid you infused. This is normal if they have trouble with swelling or pain, it is recommended that they can use a warm or cold compress, but advise your patient not to leave it on for more than ten minutes at a time.
Nurse: The swelling should go away after 1 to 3 days, but if it doesn't or begins to worsen, they should call their HCP. Other than that, patients may experience mild or moderate pain, redness and itching at the infusion site. The most common systemic side effects are headache, vomiting, fatigue, nausea, and fever. Remember, these are not all of the possible side effects.
Nurse: Again, tell your patient to contact their HCP if their reaction concerns them or does not go away. Make sure your patient is doing okay and move on to the next step. This takes us to hy five. Complete the infusion and dispose of infusion set and HyHub. At the end of the infusion check to ensure all the medicine has been infused from the IG vial or vials depending on your dose.
Nurse: If medicine remains, your patient should resume the infusion until the entire dose of IG has been infused. Next, have them remove the IG connector from the pump tubing. At this point, your patient may flush the tubing if appropriate for their infusion and directed by their HCP. They can do this by twisting a prefilled flush syringe of saline approximately five milliliters onto the tubing.
Nurse: Then they can push the syringe’s plunger to receive the entire IG dose prescribed. Once the IG connector from the pump tubing is removed, your patient can remove the needle set by loosening the tape on all edges and pulling the needle wing straight up and out. After that, they can gently press a small piece of gauze over the infusion site and cover it with the bandage.
Nurse: After removing the needle or needles from the skin, then infusion should be discarded in the sharps container. Patients should discard the HyHub infusion tray with the vials attached, and dispose of the other supplies as directed. Remind your patient that they will get a new HyHub infusion tray for every infusion. A few things to note do not remove the vials from the HyHub infusion tray before disposal.
Nurse: HyHub is for single use only. Your patient can't re sterilize HyHub and reuse will increase their risk of infection. The last thing is for your patient to record the infusion details in their wellness journal if they have one. Have your patient write down the batch numbers on the vials, as well as the date and time sites of infusion, any reactions after each infusion and any notes they want to remember or experiences they want to talk about with their treatment team.
Nurse: Congratulate your patient. They've just completed hy five. Indications. HyQvia is indicated for the treatment of primary immunodeficiency (PI) in adults and pediatric patients two years of age and older, and for chronic inflammatory demyelinating polyneuropathy CIDP as maintenance therapy to prevent relapse of neuromuscular disability and impairment in adults.
Nurse: HyQvia is for subcutaneous use only. Important safety information. Warning thrombosis. Thrombosis may occur with immune globulin IG products, including HyQvia. Risk factors may include advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling vascular catheters, hyperviscosity and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
Nurse: For patients at risk of thrombosis, administer HyQvia at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity. Contraindications. History of anaphylactic or severe systemic hypersensitivity reactions to human IG. IGA deficient patients with antibodies to IGA, and a history of hypersensitivity to human IG.
Nurse: Known systemic hypersensitivity to hyaluronidase including recombinant human hyaluronidase of HyQvia. Known systemic hypersensitivity to human albumin and hyaluronidase solution. Warnings and precautions. Hypersensitivity. Severe hypersensitivity reactions may occur after treatment with IG products, including HyQvia even in patients previously treated with IG products. If a hypersensitivity reaction occurs, discontinue infusion immediately and institute appropriate treatment. IGA deficient patients with antibodies to IGA are at greater risk of developing potentially severe hypersensitivity reactions, including anaphylaxis. Thrombosis has been reported to occur following treatment with IG products, including HyQvia, and in the absence of known risk factors and patients at risk administer at the minimum dose and infusion rate practicable.
Nurse: Ensure adequate hydration before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity. Immunogenicity of recombinant human hyaluronidase rHuPH20 non neutralizing antibodies to the recombinant human hyaluronidase component can develop. The clinical significance of these antibodies, or whether they interfere with fertilization in humans is unknown. Aseptic meningitis syndrome has been reported to occur with use of IG, including HyQvia.
Nurse: The syndrome usually begins within several hours to two days following IG treatment. Conduct a thorough neurological exam on patients exhibiting signs and symptoms to rule out other causes of meningitis. Discontinuing IG treatment has resulted in remission within several days without sequelae. Hemolysis. HyQvia contains blood group antibodies, which may cause a positive direct anti globulin reaction and hemolysis.
Nurse: Monitor patients for signs and symptoms of hemolysis and delayed hemolytic anemia, and, if present, perform appropriate confirmatory lab testing. Renal dysfunction and failure. Acute renal dysfunction and failure. Acute tubular necrosis. Proximal tubular nephropathy. Osmotic nephrosis, may occur with IG products, including HyQvia. Ensure patients are not volume depleted prior to infusion, and patients at risk due to preexisting renal insufficiency or predisposition to acute renal failure.
Nurse: Administer HyQvia at the minimum rate of infusion practicable. Assess renal function before initiation and throughout treatment, and consider lower, more frequent dosing. If renal function deteriorates, consider discontinuation. Spread of localized infection. Do not infuse HyQvia into or around an infected area due to potential risk of spreading a localized infection. Transfusion related acute lung injury. Non cardiogenic pulmonary edema may occur with I.V. administered IG.
Nurse: Monitor patients for pulmonary adverse reactions. If suspected, perform appropriate test for presence of anti neutrophil and anti HLA antibodies in both product and patient serum. Manage use in oxygen therapy with adequate ventilatory support. Transmittable infectious agents. Because HyQvia is made from human plasma, there is a risk of transmitting infection agents, e.g. viruses, other pathogens, interference with lab tests. False positive serological test results in certain assay readings with the potential for misleading interpretation may occur as a result of passively transferred antibodies.
Nurse: Adverse reactions. The most common adverse reactions observed in greater than 5% of patients in the clinical trials were primary immunodeficiency PI, local reactions, headache, antibody formation against rHUPH20, fatigue, nausea, pyrexia and vomiting. Chronic inflammatory demyelinating polyneuropathy, CIDP, local reactions, headache, pyrexia, nausea, fatigue, erythema, pruritus, increased lipase, abdominal pain, back pain and pain, and extremity. Drug interactions.
Nurse: Passive transfer of antibodies may transiently interfere with immune responses to live attenuated virus vaccines e.g. measles, mumps, rubella, and varicella. Use in specific populations. Pregnancy. Limited human data are available on the use of HyQvia during pregnancy. The effects of antibodies to the recombinant human hyaluronidase on the human embryo or fetal development are unknown. It is not known whether HyQvia can cause fetal harm when administered to a pregnant woman, or if it can affect reproductive capacity.
Nurse: HyQvia should be given to a pregnant woman only if clearly needed. Please see additional important safety information throughout this video. Accompanying full prescribing Information can be found on the HyQviahcp.com. Please discuss any questions or challenges with your patient's health care provider. HCP.
Talk to a representative
Get answers to your questions about HyQvia
HyHub/HyHub Duo Important Information for Healthcare Providers
Intended Use: HyHub/HyHub Duo are stand-alone, single-use, disposable vial access devices.
Indications for Use: HyHub/HyHub Duo are indicated for patients 17 years of age and older to allow HyQvia to be transferred from vials without using a needle, as prescribed, in a home environment or clinical setting.
Contraindications:
- Do not use HyHub/HyHub Duo with a pooling bag.
- Do not connect HyHub/HyHub Duo to a syringe driver infusion pump.
Selected Information for Patients:
- HyHub/HyHub Duo are for SINGLE USE ONLY, even if all docks are not used during a single infusion. Re-use will increase risk of infection. Patients should always use a new HyHub/HyHub Duo for each infusion.
- Only use HyHub/HyHub Duo when patients are ready to administer HyQvia.
- Patients should not use HyHub/HyHub Duo at home until receiving instructions and training from a healthcare provider.
- HyQvia is the only medication that may be used with HyHub/HyHub Duo.
- Patients should not exceed the maximum infusion volume per infusion site or infusion rate as indicated in the HyQvia prescribing information.
For safe and proper use of HyHub/HyHub Duo, please refer to the complete Instructions for Use included with the devices. For information about HyQvia, please see Prescribing Information for HyQvia.
References
- HyQvia. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- Beydoun SR, Sharma KR, Bassam BA, Pulley MT, Shije JZ, Kafal A. Individualizing therapy in CIDP: A mini-review comparing the pharmacokinetics of lg with SCig and IVig. Front Neurol. 2021;12:638816. doi:10.3389/fneur.2021.638816
- Goyal NA, Karam C, Sheikh KA, Dimachkie MM. Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2021;64(3):243-254. doi:10.1002/mus.27356
- GBS-CIDP.org Voice of the Patient. Chronic Inflammatory Demyelinating Polyneuropathy. Accessed January 2024. https://www.gbs-cidp.org/wp-content/uploads/2022/08/GBSCIDP-Voice-of-the-Patient-Report_Final.pdf
- HyHub. Instructions for use. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- HyHub Duo. Instructions for use. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- HyOvia Data on File. Reduction in number of infusion steps.
- HyQvia Data on File. CIDP ADVANCE-1 Patient Choice Data Exclude 1 site.
- Bril V, Hadden RDM, Brannagan TH 3rd, et al. Hyaluronidase-facilitated subcutaneous immunoglobulin 10% as maintenance therapy for chronic inflammatory demyelinating polyradiculoneuropathy: The ADVANCE-CIDP 1 randomized controlled trial. J Peripher Nerv Syst. 2023;28(3):436-449. doi:10.1111/ins.12573
- Hadden RDM, Andersen H, Bril V, et al. Long-term safety and tolerability of hyaluronidase-facilitated subcutaneous immunoglobulin 10% as maintenance therapy for chronic inflammatory demyelinating polyradiculoneuropathy: Results from the ADVANCE-CIDP 3 trial. J Peripher Nerv Syst. 2024;29(4):441-452. doi:10.1111/jns.12672
- HyQvia Data on File, CSR, table 14 2.5, 2.1 page 1564.
- GAMMAGARD LIQUID. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.
- GAMUNEX-C. Prescribing information. Grifols Therapeutics LLC; 2020.
- Privigen. Prescribing information. CSL Behring AG; 2025.
- Hizentra. Prescribing information. CSL Behring AG; 2023.
- Dosing and Administration, GAMUNEX-C. Accessed October 9, 2023. https://www.gamunex-c.com/en/hcp/cidp/dosing-administration
- Dosing & Infusion. Privigen. Accessed October 9, 2023. https://www.privigen.com/hcp/dosing
- van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35-46. doi:10.1016/S1474-4422(17)30378-2
- Miars LK, Tran M, Duff K. Practical considerations for self-administration of subcutaneous immunoglobulin G utilizing recombinant human hyaluronidase: a nurse’s perspective. J Infus Nurs. 2016;39(6):359-368. doi:10.1097/NAN.0000000000000182
- Moog Medical. CURLIN Infusion Administration Sets. Accessed October 25, 2022. https://www.moogmedical.com/infusion/sets/
- Sapphire Multi-therapy and Dedicated Infusion Pumps. User Manual. Rev. 15. Eitan Group and Q Core Medical Ltd.; 2019.
 , and
, and