subcutaneous
administration


One monthly* dose.1
Flexible administration.1
HyQvia delivers long-lasting,† monthly* protection for your patients with PI who want a little more time between infusions.

*Every 3 or 4 weeks.
Median infusion time was 2.08 (0.83-4.68) hours vs 2.33 (0.92-6.33) for IVIG in the clinical trial.
†Between infusions.
With HyQvia, monthly* SCIG dosing could mean fewer infusions for your patients1
Number of infusions per year
50%
Patients’ number of infusions per year could be reduced by 50% or more
*Every 3 or 4 weeks.
cSCIG=conventional subcutaneous immune globulin.
Hyaluronidase is dosed at a fixed ratio of 0.5 mL rHuPH20 solution per 10 mL Immune Globulin Infusion 10% (Human) solution. This is supplied as part of a dual unit vial. Administer the full content of the Hy vial/s for both a full and partial dose of IG.
HyQvia dosing information
HyQvia [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] comes in a dual vial unit—one vial of Immune Globulin Infusion 10% (Human) Solution [IG 10%] and one vial of Recombinant Human Hyaluronidase.1
The HyQvia dose is based on the immune globulin (IG 10%) component, which provides the therapeutic effect. Dispersion and absorption of the 10% IG is facilitated by the Recombinant Human Hyaluronidase.1
HyQvia initial monthly* dosing (following ramp-up) may vary depending on whether patients are switching from intravenous immune globulin (IVIG) treatment or conventional subcutaneous immune globulin (SCIG) treatment.1
*Every 3 or 4 weeks.
HyQvia dosing calculator
Determine dosage range
To determine the dosage ranges for a patient, select the weight closest to the patient’s weight in the window on the left. Dosage ranges will appear in grams. See Full Prescribing Information for additional dosage recommendations. A conversion factor of 2.2046 lb/kg is used to convert the patient’s weight to kilograms. The values displayed are rounded to the nearest decimal point.
What is the patient's weight?
Dosage range to be prescribed every 3-4 weeks or per patient needs
This calculator does not represent every possible weight calculation. Weights begin as 24 lb and increase in 4 lb increments to 288 lb.
Select available HyQvia vial combinations based on dosage
When available HyQvia solution vial sizes cannot be combined to equal the calculated IG dose, the dose must be rounded up or down.
The Physicians should make this determination based on their clinical judgment and HyQvia vial sizes.
HyQvia doses, based on available vial combinations, are shown in the table below.
| 2.5 | 5 | 7.5 | 10 |
| 12.5 | 15 | 17.5 | 20 |
| 22.5 | 25 | 27.5 | 30 |
| 32.5 | 35 | 37.5 | 40 |
| 42.5 | 45 | 47.5 | 50 |
| 52.5 | 55 | 57.5 | 60 |
| 62.5 | 65 | 67.5 | 70 |
| 72.5 | 75 | 77.5 | 80 |
*HyQvia is available in 2.5, 5, 10, 20, and 30 g vials of IG 10%. This table shows possible vial combinations at increasing 2.5 g increments, reading from left to right and top to bottom.
Switching patients to HyQvia
When switching to HyQvia, dose and frequency are determined by whether a patient is switching from an IVIG or a SCIG.1
Patients switching from IVIG treatment
Same dose and frequency of IVG
(after initial ramp-up)
Patients switching from SCIG
300 to 600 mg/kg at 3- or 4-week intervals
(after initial ramp-up)
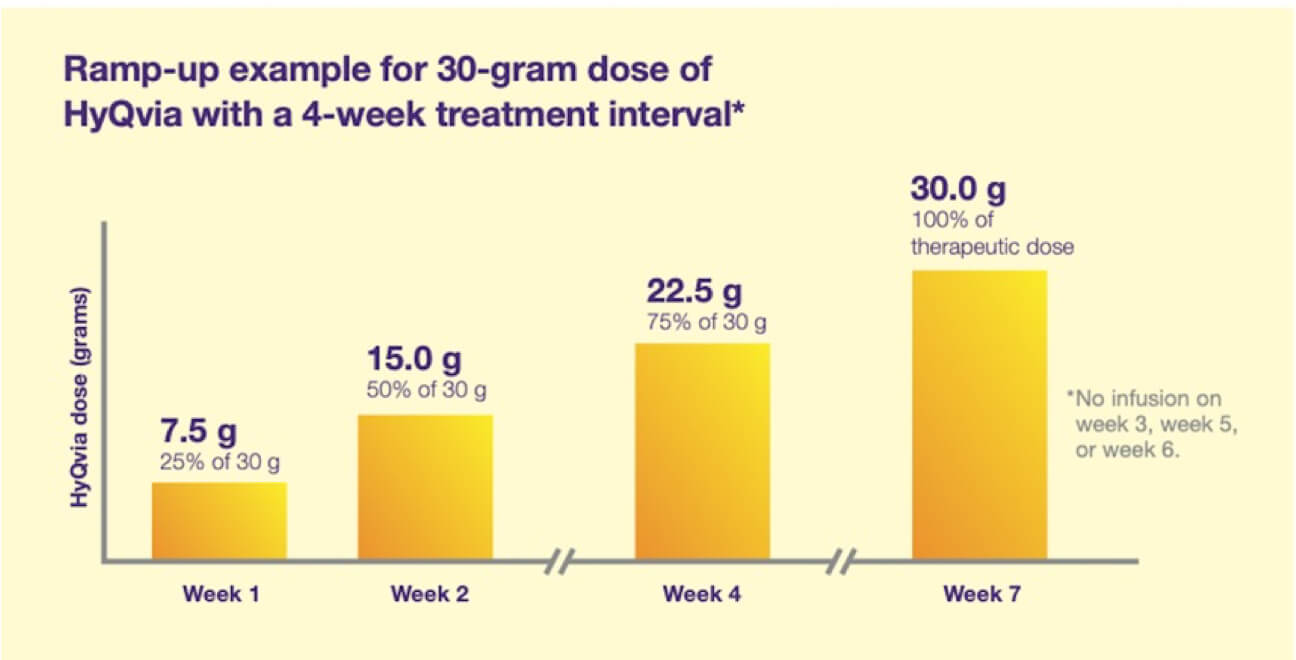
How to ramp up HyQvia
There is a ramp-up period for HyQvia which allows patients to become familiar with the volumes required for a full 3- or 4-week treatment.1
Initial treatment and HyQvia ramp-up schedule
Treatment with HyQvia is initiated by gradually increasing the dose and decreasing the frequency from a 1-week dose to a 3- or 4-week dose.
Dosage during this ramp-up period is calculated by dividing the total 3- or 4-week therapeutic dose as shown in the graph.
The first dose of HyQvia should be given approximately 1 week after the last infusion of the previous treatment.
To access patient resources, visit Access & Support.
For more comprehensive information on HyQvia dosing, see Full Prescribing Information
Infusion experience
Over the course of the efficacy and extension trials of HyQvia in ~3.5 years (2959 infusions), there were no clinically observable changes in the skin or subcutaneous tissue.1
Individual results may vary.
Pre-Infusion
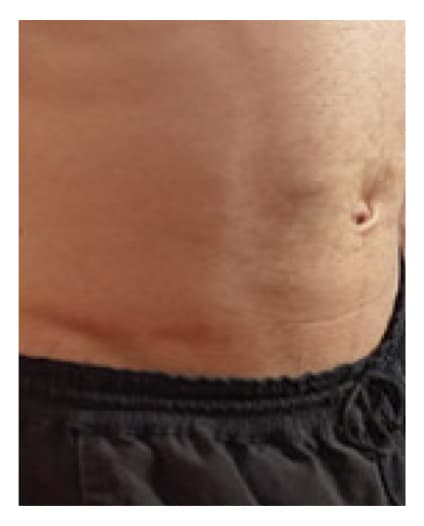
Patient weight: 168 lb
Volume: 500 mL
Number of Infusion sites: 1
Post-Infusion
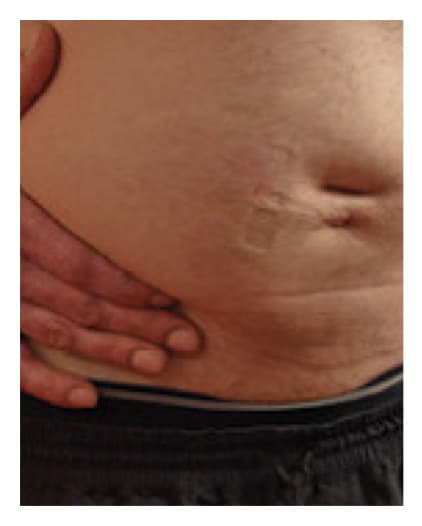
Results in a diffuse, temporary, localized swelling
24 Hours Post-Infusion
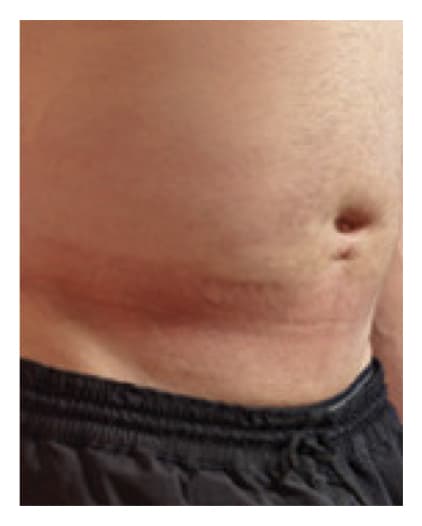
Infusion site swelling generally resolved within 1 to 3 days
Pre-Infusion
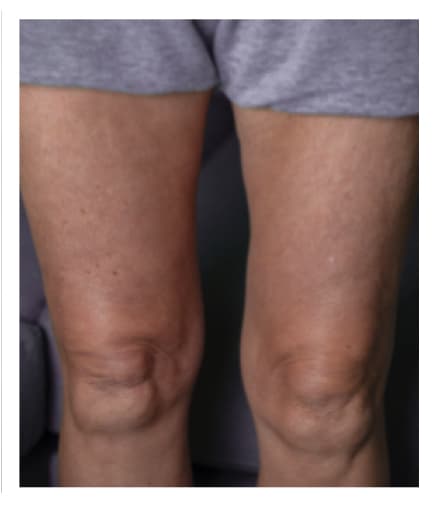
Patient weight: 162 lb
Volume: 300 mL
Number of Infusion sites: 2
Post-Infusion
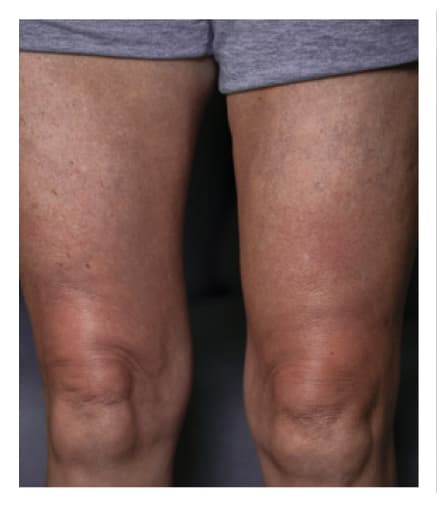
Side effects include acute tenderness
24 Hours Post-Infusion

Infusion site swelling generally resolved within 1 to 3 days
Infusion time estimator
See how HyQvia patients could spend less than half the time infusing compared to conventional IVIG1
How long do they spend infusing?
Monthly dose (in grams)
monthly
1 site
2 sites
*Every 3 or 4 weeks.
Flexible administration options1
You can work with your patients to determine:
- Either 1 or 2 infusion sites
- A second site can be used at the discretion of the physician and patient based on tolerability and total volume
- Infusion site location in either the abdomen or the thighs
- Every 3- or 4-week treatment intervals
- Infuse at home, after adequate training, or in-center
Infusion preparation options for your patients
To better meet the needs of your patients, there are 3 methods available to infuse HyQvia.1 Select pumps are available for each.
Download our guide for a full list of infusion pumps and ancillary devices compatible with each method.
Patients will receive one of the following from their specialty pharmacy:
HyHub and HyHub Duo with peristaltic pump
Meet HyHub™ and HyHub™ Duo for HyQvia—infusion trays designed to help simplify the infusion preparation process for your patients 17 and older, as prescribed, without the use of transfer needles by reducing the number of steps required to prepare Hy and IG.2-4*
After the patient has trained with an infusion nurse, the specialty pharmacy will send the tray that accommodates the prescribed dose free of cost.
*Compared to preparing with a pooling bag using 2-4 dual-vial units.2
- Reduce steps by ~half—compared to preparing Hy and IG with 3 dual-vial units using a pooling bag2
- Fewer ancillary supplies—reduced by about a quarter to a half compared to preparing Hy and IG with a pooling bag5
- Simplified preparation setup—no pooling bag required to transfer IG from vials3,4
- No transfer needles during preparation of Hy and IG—HyHub allows hyaluronidase (hy) and immune globulin (IG) to be withdrawn directly from the docked vials3,4
- No additional out-of-pocket costs for HyQvia patients


HyHub/HyHub Duo Important Information for Healthcare Providers
Intended Use: HyHub/HyHub Duo are stand-alone, single-use, disposable vial access devices.
Indications for Use: HyHub/HyHub Duo are indicated for patients 17 years of age and older to allow HyQvia to be transferred from vials without using a needle, as prescribed, in a home environment or clinical setting.
Contraindications:
- Do not use HyHub/HyHub Duo with a pooling bag.
- Do not connect HyHub/HyHub Duo to a syringe driver infusion pump.
Selected Information for Patients:
- HyHub/HyHub Duo are for SINGLE USE ONLY, even if all docks are not used during a single infusion. Re-use will increase risk of infection. Patients should always use a new HyHub/HyHub Duo for each infusion.
- Only use HyHub/HyHub Duo when patients are ready to administer HyQvia.
- Patients should not use HyHub/HyHub Duo at home until receiving instructions and training from a healthcare provider.
- HyQvia is the only medication that may be used with HyHub/HyHub Duo.
- Patients should not exceed the maximum infusion volume per infusion site or infusion rate as indicated in the HyQvia prescribing information.
For safe and proper use of HyHub/HyHub Duo, please refer to the complete Instructions for Use included with the devices. For information about HyQvia, including warnings for thrombosis, please see Prescribing Information for HyQvia.
Watch our HyHub for HyQvia overview video:
- 0:00 Nurse training video for HyHub
- 5:55 hy1: Gather & prepare for infusion
- 15:02 hy2: Prepare the infusion site
- 16:05 hy3: Infuse hyaluronidase, or Hy
- 17:48 hy4: Infuse immune globulin, or IG
- 20:01 hy5: Complete & log infusion
Peristaltic pump and pooling bag
Hy component is infused by manual syringe push. IG component is infused by the peristaltic pump.
Download our brochure on how to infuse HyQvia with a peristaltic pump.
Watch our peristaltic pump infusion video:
- 0:00 Get familiar with hy5
- 3:35 hy1: Get ready
- 4:31 hy2: Prepare the hyaluronidase, or Hy
- 6:42 hy3: Prepare the immune globulin, or IG
- 10:37 hy4: Infuse
- 15:30 hy5: Finish up
What is HyQvia [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase]?
HyQvia is a liquid medicine that is given under the skin (subcutaneously) to treat primary immunodeficiency (PI) in people 2 years and older.
Important safety information
What is the most important information that I should know about HyQvia?
- HyQvia can cause blood clots.
- Call your healthcare professional (HCP) if you have pain, swelling, warmth, redness, or a lump in your legs or arms, other than at the infusion site(s), unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body.
- Your HCP may perform blood tests regularly to check your IgG level.
- Do not infuse HyQvia into or around an infected or red swollen area because it can cause infection to spread.
Please see additional Important Safety Information throughout this video and accompanying Information for Patients found on this website, and discuss with your healthcare provider.
Nurse: Hi! I’m Lisa, and I’m a nurse. I’m here with Jackson to help him learn how to self-administer his HyQvia treatment, which just means after he is trained, he’ll be able to infuse at home.
Nurse: Are you ready?
Patient: I think so, but I am a little nervous.
Nurse: That’s normal. I’m going to walk you through everything. And with proper training, you will be able to do this. So, I see you’ve got some supplies here.
Patient: Yeah, I think this is everything. It’s…a lot.
Nurse: I know. It IS a lot, and I’m sure it was a bit overwhelming looking at all of this for the first time, but that’s why I’m here. It’ll all make sense. And once you do this a few times, you’ll have it down. You can always reach out to your doctor if you have questions or concerns.
Nurse: Let’s start with the obvious. Here are your HyQvia vials. The number of vials each person will have will depend on their dose. This guy’s your pump and its tubing. You’ve got an IV pole and syringe. This is your pooling bag and tubing. Here’s your sharps container. This is what you put things in, like your needles, to dispose of when you’re done. We’ll get to that at the end of the infusion. And you’ve got a Wellness Journal here. This helps you track your treatments and has space to write down any notes or questions. We’ll get to this at the end of your infusion, too.
Nurse: OK, these are your gloves, obviously. You’ll wear these if your doctor recommends that you should. Here’s a bag of saline solution, needles, needle set, tape, bandages, alcohol swabs to clean the tops of your vials and your body where you infuse.
Nurse: I just said a ton, and I can see the look on your face. What are you thinking?
Patient: That’s…so much, but I think I caught most of it.
Nurse: I understand. I know it’s a lot. You’ll start to remember everything. So, now that we know you’ve got everything, ready to get started?
Patient: I’m still a little nervous, but let’s do it.
Nurse: Great. To help remember how to infuse HyQvia, we use hy5, like the hy in both HyQvia and hyaluronidase, this little vial.
Nurse: Oh, good. Your vials are at room temperature, just like they should be. Vials can be stored in the refrigerator or at room temperature.
Nurse: They do last longer if they’re refrigerated. But, if they’ve been refrigerated, the HyQvia may take up to 60 minutes when taken out of the boxes to get to room temperature. Just something to remember to help plan when you’ll infuse.
Nurse: Always let it come to room temperature naturally. So, don’t ever apply heat or place in the microwave.
Patient: Got it.
Nurse: Before we dig in, let’s take a look at your vials.
Nurse: You always want to make sure they’re the correct dose, what your doctor prescribed for you.
Nurse: Check the expiration date and make sure they’re not expired. Don’t ever use them if they’re past the expiration date or if the purple cap is missing or broken. You want to handle them normally. So, no shaking. Look at the color. The Hy should be clear and colorless. And the IG should be clear and colorless, or it may be pale yellow. That’s OK. Neither should be cloudy or have any floating particles.
Patient: OK, these look OK, right?
Nurse: Yep! They look good to me. We just need to finish gathering our supplies, clean our work area, wash our hands, and we’re ready to move on.
Nurse: OK, let’s wash our hands and we’re ready for hy2 of hy5: Preparing the Hy.
Patient: OK, sounds good. The sink’s right over here.
Nurse: Now when we say “Hy,” we’re talking about the recombinant human hyaluronidase.
Nurse: It is always first, so it’s infused before the IG, and Hy allows your body to absorb more IG, your entire dose, into the bloodstream in one or two sites subcutaneously.
Nurse: Remove the purple protective cap and ensure the purple vial caps are removed. To prepare the Hy, first wipe the vial with an alcohol swab and give it a few seconds to dry.
Nurse: Next, remove a sterile syringe from its package and attach it to the needle or needleless transfer device, like this.
Nurse: Next, pull back on the plunger to fill the syringe with air. The amount of air should equal the amount of Hy in the vial.
Nurse: Then, insert the needle or needleless transfer device into the center of the Hy vial stopper and push the air into the vial.
Nurse: Now, turn the vial upside down and pull back on the plunger to withdraw all of the Hy into the syringe.
Patient: Like this?
Nurse: Yes, you’ve got it.
Nurse: Now, remove the needle from the Hy vial and carefully recap it. If you have multiple vials of HyQvia, repeat the previous steps using a new needle or needless transfer device for each vial to collect all of the Hy into a single syringe.
Nurse: Great! So, we’ve got all the Hy we need into the syringe. Now to attach it to the needle set. First thing we’ll want to do is tap the syringe to remove any air bubbles.
Nurse: Then push the syringe’s plunger until you see that the Hy reaches the tip of the barrel. Like this. See?
Patient: Yep. Got it.
Nurse: Now, let’s attach the subcutaneous needle set to the syringe by first removing the needle from the syringe. Next, remove the white cap from the subcutaneous needle set. Then, attach the syringe to the needle set. Slowly push the plunger to fill the needle set tubing, stopping before the Hy reaches the needle hub so it doesn’t coat the needle. Yep. OK, good.
Nurse: Lay the syringe down on the clean work surface and clamp the needle set tubing. Guess what? You’re already to hy3.
Patient: OK!
Nurse: hy3 is preparing the IG infusion 10% (human)—that’s your IG full of all those antibodies to help you fight off infections. First, clean each vial by wiping the stopper with an alcohol swab and give it a few seconds to dry.
Nurse: Take the pooling bag out and close off all of these white clamps on the tubes that are coming out of the pooling bag. Make sure to keep this little cap nearby. Here, it’s in sterile packaging, but it may not always be. That’s OK. You would then want to keep it on a sterile and clean surface. OK, do you see where the pooling bag connects to the tubes? That’s the port.
Patient: Yes, this little white piece?
Nurse: Yep, right there. You want to make sure that’s tight because sometimes it can become loose in the packaging.
Patient: Got it. It’s tight.
Nurse: Open and close the vent on the pooling bag tubes to allow it to open easier.
Nurse: Now, remove the cap from the spike on one of the pooling bag tubes.
Nurse: Insert the spike straight down into the center of the IG vial stopper. If you have multiple HyQvia vials, you’ll use multiple IG spikes. The tip of the spike should be all the way inside the stopper.
Patient: OK, it is now.
Nurse: Great! Now turn the IG vial upside down and open the clamp on the pooling bag tube and then open the vent. You may need to gently flick the pump set tubing to float air bubbles to the top of the vial.
Patient: Like this?
Nurse: Exactly!
Nurse: Keep holding the IG vial upside down and transfer the IG into the pooling bag. Then, close the clamp and the vent on the pooling bag tubing. If additional vials of IG are needed in order to reach your prescribed dose, repeat the steps for spiking each additional IG vial as needed. When you’ve finished with the last vial, hold the pooling bag tubing upright.
Nurse: Tap the pooling bag tubing to get the last drops of IG into the pooling bag.
Patient: Whoa, OK, I was with you up until when you talked about when I’m finished with the last vial.
Nurse: That was a lot. No worries. OK, once the last vial is finished, you want to hold the pooling bag tubing upright. Tap the pooling bag tubing to get the last drops of IG into the pooling bag.
Patient: OK, got it.
Nurse: Good. Hold the pooling bag so the tubes are coming out of the top and then detach the pooling bag tubes from the pooling bag. Now, grab that cap and twist it onto the port we pointed out before.
Patient: Is this right?
Nurse: Just like that! Next, close the clamp on the pooling bag port. And then reach for your pump tubing, and go ahead and take that out of the package. Close the clamp on the pump tubing.
Nurse: Remove the cap from the administration port of the pooling bag. On your pooling bag, that’s this middle port.
Nurse: Now, remove the cap from the spike on the pump tubing.
Nurse: Insert the spike into the administration port of the pooling bag. Then, with a twisting motion, insert the pump tubing into the pump to fill the pump tubing.
Nurse: Hang the pooling bag onto an IV pole and follow instructions from the pump manufacturer of how to fill the pump tubing with the IG to the end of the tubing.
Nurse: OK, so now we’ve prepared both the Hy and IG. Ready to infuse your HyQvia?
Patient: I am.
Nurse: Yes, that’s the attitude I’m talkin’ about.
Nurse: On to hy4: Infusing HyQvia. The Hy and the IG will be infused differently.
Nurse: We’ll infuse the Hy manually and then the IG with the peristaltic infusion pump. But always in that order. Remember, Hy before IG.
Patient: Yes, I’ll remember.
Nurse: OK, we’ll start by choosing an infusion site. Where are you thinking?
Patient: Well…I’m…not exactly sure. I was kind of confused about that. Where should I?
Nurse: You’ve got two choices. You can infuse in either thigh or either side of your middle to upper abdomen. And the site can change over time after you’ve infused a few times and realized one area is more comfortable for you than another.
Patient: I think I’d like my abdomen.
Nurse: That will work! Remember to rotate your sites for each infusion. You can choose to infuse at two sites to help spread out the volume across multiple parts of the body. This potentially allows a faster infusion time than if just one site is used. A second site can be used at the discretion of your physician and based on tolerability and total volume. If a second site is used, you want to select sites on the opposite sides of your body and infuse half the total volume of Hy in each site.
Nurse: OK, use a new alcohol swab to clean your abdomen and let it dry completely. Good.
Nurse: Now, I want to make sure I mention a few things about the needles. You want to avoid any bony areas, blood vessels, scars, and any areas of inflammation, irritation, or infection. Your goal is to insert the needle just under the skin, into the fatty layer. Now, if the needle is too short, you may experience a burning sensation or leakage, but, on the other hand, if it’s too long, you’ll reach the muscle layer, and you don’t want to do that. If you feel that you need a needle of a different length, speak with your doctor and reach out to your specialty pharmacy, because that’s where you can get the new needle or needles.
Nurse: Remove the needle cap and firmly grasp and pinch at least one inch of skin between two fingers. Insert the needle with a rapid motion straight into your skin at a 90-degree angle. Alright, now you just gotta do it!
Nurse: Yes! Just like that. You did it. You OK?
Patient: Surprisingly, yes.
Nurse: You did great. Now, secure the needle in place with the sterile tape.
Nurse: Great. Ready to start?
Patient: I’ve come this far. Let’s do it.
Nurse: OK, here we go! Open the clamp on the needle set and gently pull back on the syringe plunger. Look for blood return in the tubing. If there’s no blood, you can secure the needle set by applying a sterile, clear dressing over the site. If more than one infusion site is needed, you'll follow these steps to check for proper needle placement in both sites at the same time.
Nurse: If blood is seen in the tubing, remove and discard the needle and start at a new site with a new subcutaneous needle.
Infuse the Hy at a rate of one to two milliliters per minute, per infusion site and increase as tolerated.
Nurse: When all the Hy has been infused, detach the syringe from the needle set, but don’t remove the needle from your infusion site because we’re going to attach the same needle set to the pump tubing. Remove the sterile cap on the pump tubing and twist the pump tubing, filled with IG, to the right onto the same needle set.
Patient: Ah, OK.
Nurse: Now, open the clamp on the pump tubing and start the pump. There you go: You’re infusing the IG. Just make sure to start the IG infusion right after the Hy infusion is complete, within 10 minutes at the most.
Nurse: I’m sure at some point you’ve heard or read about ramping up, right?
Patient: Yes, my doctor talked to me about it. She said I’d start with a lower volume and rate and increase for the first four infusions.
Nurse: That’s exactly right. It’s a way to help your body adjust to your new medicine.
Nurse: So when the IG infusion is complete, flush any remaining IG from the pump tubing with saline to ensure that you receive your full dose. To do that, first remove the pump tubing spike from the pooling bag administration port. Now, pull the tab off the saline bag administration port and insert the pump tubing spike into the administration port. Finally, restart the pump to flush the remaining IG from the pump tubing.
Nurse: So this whole infusion process from start to finish typically takes about 3 to 4 hours, but as far as setting up your infusion goes, that’s a wrap! You did it.
Patient: You mean that’s it?
Nurse: For the infusion, yes.
Nurse: We’re at hy5: Finish up.
Nurse: You’re going to loosen the clear dressing and pull the needle wings straight up and out. Place a bandage over the infusion site. Then, dispose of the needle set in the sharps container.
Nurse: I do have to say, some patients experience a slight bit of swelling. They call it a “pancake” because your skin swells in the shape of a little pancake. However, this is common and nothing to panic about. This means the Hy and IG are doing what they’re supposed to do. It will go down in one to three days, due to the volume of fluid infused.
Patient: That sounds a little weird, but I’m definitely glad you told me about it, so I know what to expect.
Nurse: I know it does, but I want you to know and not be surprised if it happens. It’s common.
Nurse: Other than that, you may experience local site reactions, like mild or moderate pain, redness, swelling, and itching at the infusion site. The most common general side effects, also known as systemic side effects, are headache, vomiting, fatigue, nausea, and fever. Keeping in mind these are not all the possible side effects of HyQvia. Talk to your doctor about any side effect that bother you or do not go away.
Nurse: I’m sure you’ve heard or read about side effects, but just making sure we go over them too. You want to contact your doctor if a reaction concerns you or does not go away.
Nurse: Now, you’ll need to record your infusion details in your Wellness Journal. You want to write down the date, time, dose, and infusion site(s) to assist in rotating sites and any reactions after each infusion. A trick for your Wellness Journal, most patients just pull the labels right off their IG vials, which have the product lot number, vial size, and expiration date, and put them in their journal. There’s even space to write down questions and thoughts, like how long a certain step took or just notes about remembering what to do. It’s a good place to write down any questions you may have for your healthcare team as well.
Nurse: You did it! You just learned how to use hy5 as a way to help remember how to infuse HyQvia. Seriously, you did great. I hope you’re proud of yourself.
Patient: I am. I was so nervous, but I think I can do this. Thank you so much.
Nurse: Of course! I know you’ve got this. You can have more training, depending on how comfortable you and your nurse feel about your progress. You also have resources to rely on, should you need them. And, if you have questions about anything, talk to your doctor. You can also visit HyQvia.com.
Patient: Yeah, I will.
Nurse: Now, I’ll help you gather this stuff up so it’s ready to go next time, and then you take it easy the rest of the day.
Patient: Thanks!
What is HyQvia?
HyQvia is a liquid medicine that is given under the skin (subcutaneously) to treat primary immunodeficiency (PI) in adults.
IMPORTANT SAFETY INFORMATION
What is the most important information that I should know about HyQvia?
- HyQvia can cause blood clots.
- Call your healthcare professional (HCP) if you have pain, swelling, warmth, redness, or a lump in your legs or arms, other than at the infusion site(s), unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body.
- Your HCP may perform blood tests regularly to check your IgG level.
- Do not infuse HyQvia into or around an infected or red swollen area because it can cause infection to spread.
Who should not take HyQvia?
Do not take HyQvia if you:
- Are allergic to IgG, hyaluronidase, other blood products, or any ingredient in HyQvia.
What should I avoid while taking HyQvia?
- HyQvia can make vaccines (like measles/mumps/rubella or chickenpox vaccines) not work as well for you. Before you get any vaccines, tell your HCP that you take HyQvia.
What should I tell my HCP before I start using or while using HyQvia?
Before starting HyQvia, tell your HCP if you:
- Have or had any kidney, liver, or heart problems or history of blood clots because HyQvia can make these problems worse.
- Have IgA deficiency or a history of severe allergic reactions to IgG or other blood products.
- Are pregnant, trying to become pregnant or are breast feeding. It is not known whether HyQvia can harm the unborn baby or breastfed infant.
What are the possible or reasonably likely side effects of HyQvia?
HyQvia can cause serious side effects. If any of the following problems occur after starting HyQvia, stop the infusion immediately and contact your HCP or call emergency services:
- Hives, swelling in the mouth or throat, itching, trouble breathing, wheezing, fainting or dizziness. These could be signs of a serious allergic reaction.
- Bad headache with nausea, vomiting, stiff neck, fever, and sensitivity to light. These could be signs of irritation and swelling of the lining around your brain.
- Reduced urination, sudden weight gain, or swelling in your legs. These could be signs of a kidney problem.
- Pain, swelling, warmth, redness, or a lump in your legs or arms, other than at the infusion site(s). These could be signs of a blood clot.
- Brown or red urine, fast heart rate, yellow skin or eyes. These could be signs of a liver or blood problem.
- Chest pain or trouble breathing, blue lips or extremities. These could be signs of a serious heart or lung problem.
- Fever over 100°F. This could be a sign of an infection.
After HyQvia infusion a temporary, soft swelling may occur around the infusion site, which may last 1 to 3 days, due to the volume of fluid infused. The following possible side effects may occur at the site of infusion and generally go away within a few hours, and are less likely after the first few infusions.
- Mild or moderate pain
- Redness
- Swelling
- Itching
The most common side effects of HyQvia are:
- Headache
- Fatigue
- Nausea
- Fever
- Vomiting
Antibodies to the hyaluronidase component of HyQvia were formed in some patients taking HyQvia. It is not known if there is any long-term effect. In theory, these antibodies could react with your body’s own hyaluronidase (PH20). PH20 is present in the male reproductive tract. So far, these antibodies have not been associated with increased or new side-effects.
These are not all the possible side effects. Talk to your HCP about any side effect that bothers you or that does not go away.
Please see additional Important Safety Information throughout this video and accompanying Information for Patients found on this website, and discuss with your healthcare provider.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Syringe driver pump
Hy component is infused by manual syringe push or the syringe driver pump. IG component is infused by the syringe driver pump.
A range of syringe driver pumps meet the criteria to administer HyQvia.
Download our brochure on how to infuse HyQvia with a syringe driver pump.
References
- HyQvia. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- Data on file. HyHub DOF, Reduce Prepare Hy IG Steps. 06/2025.
- HyHub. Instructions for Use. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- HyHub Duo. Instructions for Use. Takeda Pharmaceuticals U.S.A., Inc.; 2025.
- Data on file. HyHub DOF, Reduce Supply Prepare Hy IG. 06/2025.
 , and
, and  are trademarks or registered trademarks of Takeda Pharmaceutical Company Limited. HYQVIA is a registered trademark of Baxalta Incorporated. HYHUB and MYIGSOURCE are trademarks of Baxalta Incorporated.
are trademarks or registered trademarks of Takeda Pharmaceutical Company Limited. HYQVIA is a registered trademark of Baxalta Incorporated. HYHUB and MYIGSOURCE are trademarks of Baxalta Incorporated.